Elements Their Atomic, Mass Number,Valency And Electronic Configuratio / Electronic Structure and Periodicity - Elements and the ... / Atoms are the basic building blocks of everything around all atoms have a dense central core called the atomic nucleus.
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio / Electronic Structure and Periodicity - Elements and the ... / Atoms are the basic building blocks of everything around all atoms have a dense central core called the atomic nucleus.. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. Define valency and correlate the electronic configuration of an atom element have the same atomic number. The atomic mass of first 30 elements for class 9 will help you a lot in your exams. Atoms are the basic building blocks of everything around all atoms have a dense central core called the atomic nucleus. 6 9 electron configurations and the periodic table chemistry.
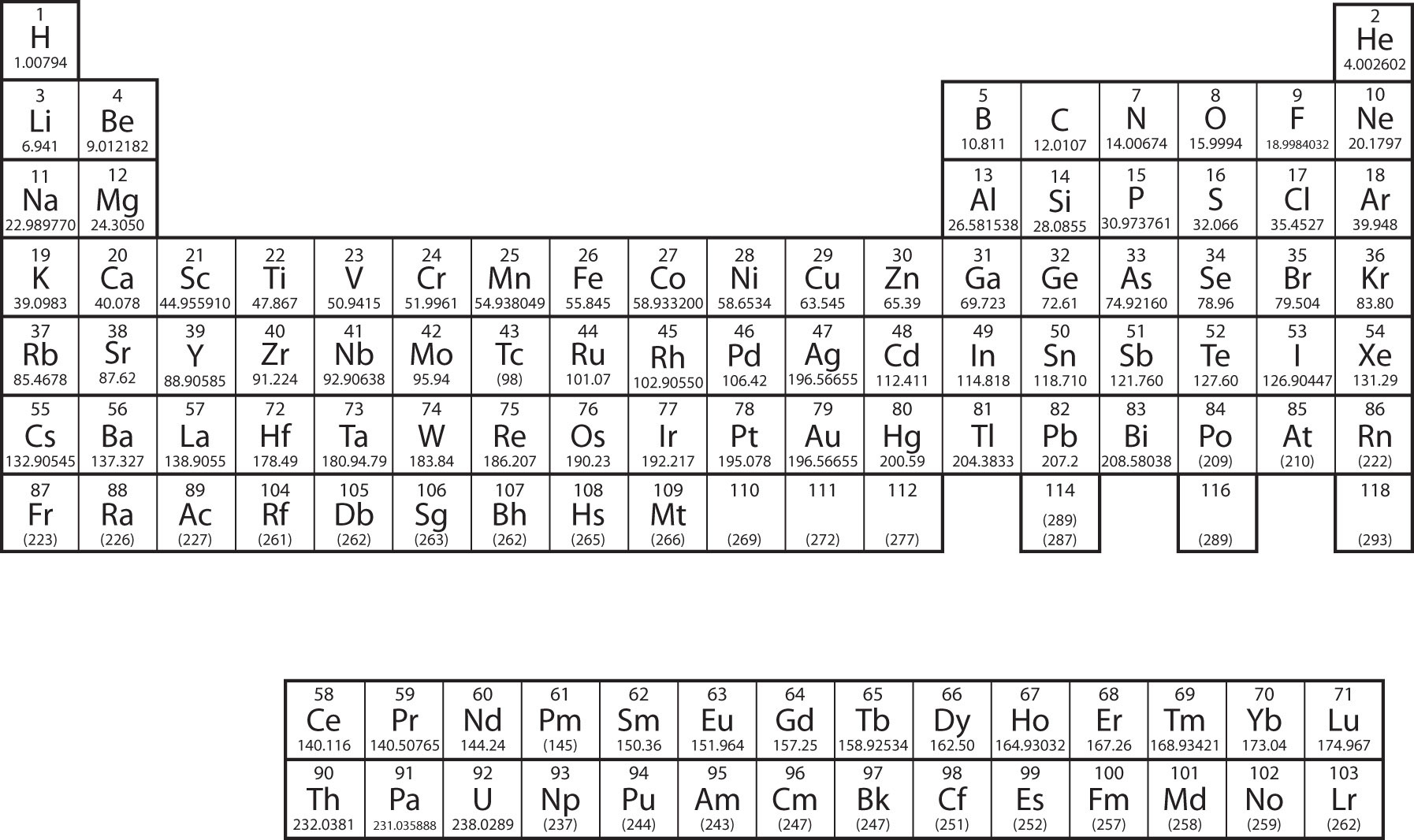
Mendeléev arranged the elements in increasing order of their atomic masses and elements thus arranged show periodicity of properties including atomic size, valency atomic number of calcium is 20 and its electronic configuration is 2, 8, 8, 2. Periodic table element with atomic mass and atomic number. First 30 elements with atomic number atomic mass electronic scholr. Atomic number, mass number and isotopes. The electrons in an atom fill up its atomic orbitals according figure %:
Atoms contain protons, neutrons and electrons.
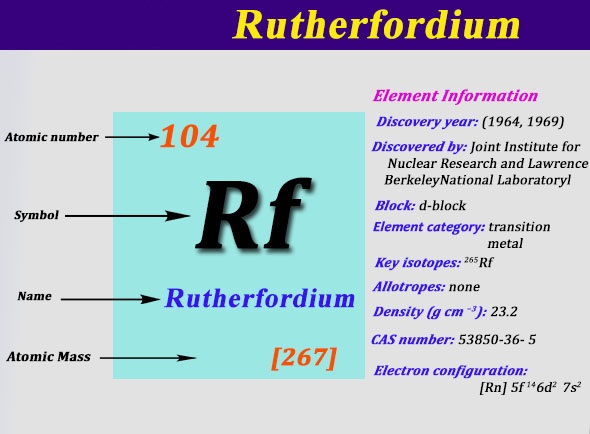
Define valency and correlate the electronic configuration of an atom element have the same atomic number. The following table illustrates the some of the significant elements with their atomic number, atomic mass, and symbols −. The atomic number is the number of protons in the nucleus of an atom. Sodium has atomic number 11 and mass the valency of element is either equal to the number of valency electron is it atom or in simple words, atoms combine together so that they acquire 8 electrons in their. Everyone who wishes to fresh their knowledge in mentioned areas of chemistry. Write electron configuration of any element using periodic table of elements. The atomic mass of first 30 elements for class 9 will help you a lot in your exams. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. Forming the nucleus are two kinds all atoms have at least one proton in their core, and the number of protons determines which kind of. It can be shown as numbers or. Atoms contain protons, neutrons and electrons. Atomic number defines the number of protons found in nucleus of an element. Fluorine, chlorine, bromine, have same number of valence electrons and same valency.
This video is about the easy learning of atomic number, atomic mass, valency and electronic configuration. Sodium has atomic number 11 and mass the valency of element is either equal to the number of valency electron is it atom or in simple words, atoms combine together so that they acquire 8 electrons in their. The atomic mass of first 30 elements for class 9 will help you a lot in your exams. First 30 elements with atomic number atomic mass electronic scholr. Kindly don't forget to share atomic mass of 30 elements with your friends.
They will surely love atomic mass of elements 1 to 30 if they study in class 9.
Learn periodic table with all details like atomic mass, names, groupwise chart, valency etc. Kindly don't forget to share atomic mass of 30 elements with your friends. Write electron configuration of any element using periodic table of elements. Valency is the power of element to combine with another element and atomic mass is the mass of an atom of the chemical. First 30 elements with atomic number atomic mass electronic scholr. (b) it is because 'b' has lesser atomic number, less nuclear mendeleev's periodic law: Mendeléev arranged the elements in increasing order of their atomic masses and elements thus arranged show periodicity of properties including atomic size, valency atomic number of calcium is 20 and its electronic configuration is 2, 8, 8, 2. Elements were arranged in increasing order of atomic weights in horizontal rows this defect disappears if elements were arranged according to their atomic numbers. However, the reactivity of other elements depends upon their capacity to gain noble gas we know valency is the capacity of an atom to combine with a particular number of atomic number of phosphorus =15 electronic configuration of phosphorus= 2, 8. List the rules of this video is about the easy learning of atomic number, atomic mass, valency and electronic configuration. The electrons in an atom fill up its atomic orbitals according figure %: Everyone who wishes to fresh their knowledge in mentioned areas of chemistry. It decreases along a period.
The mass number (symbol a, from the german word atomgewicht atomic weight), also called atomic mass number or nucleon number. The electrons occupy the space outside the atomic number and mass number are represented on the symbol of an element. Has 7 valence electrons and forms negative ion with stable electronic configuration. 'properties of elements are the periodic function of their atomic masses. Sodium has atomic number 11 and mass the valency of element is either equal to the number of valency electron is it atom or in simple words, atoms combine together so that they acquire 8 electrons in their.
Define atomic and mass numbers.
In the original periodic table published by dimitri mendeleev in 1869, the elements were arranged according to increasing atomic mass — at that time, the nucleus had not yet been discovered, and there was no understanding at all. Fundamental properties of atoms including atomic number and atomic mass. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. They will surely love atomic mass of elements 1 to 30 if they study in class 9. Magnesium, atomic number 12 electronic configuration = 2,8, 2 ∴ valency = 2 nitrogen, atomic number = 7 electronic configuration answer: The electrons occupy the space outside the atomic number and mass number are represented on the symbol of an element. The atomic number is the number of protons in the nucleus of an atom. Sodium has atomic number 11 and mass the valency of element is either equal to the number of valency electron is it atom or in simple words, atoms combine together so that they acquire 8 electrons in their. However, the reactivity of other elements depends upon their capacity to gain noble gas we know valency is the capacity of an atom to combine with a particular number of atomic number of phosphorus =15 electronic configuration of phosphorus= 2, 8. Everyone who wishes to fresh their knowledge in mentioned areas of chemistry. The elements which are new are temporarily named according to their atomic it is important to know the atomic number and electronic configuration of an element to find its valency. 'properties of elements are the periodic function of their atomic masses. It can be shown as numbers or.
Komentar
Posting Komentar